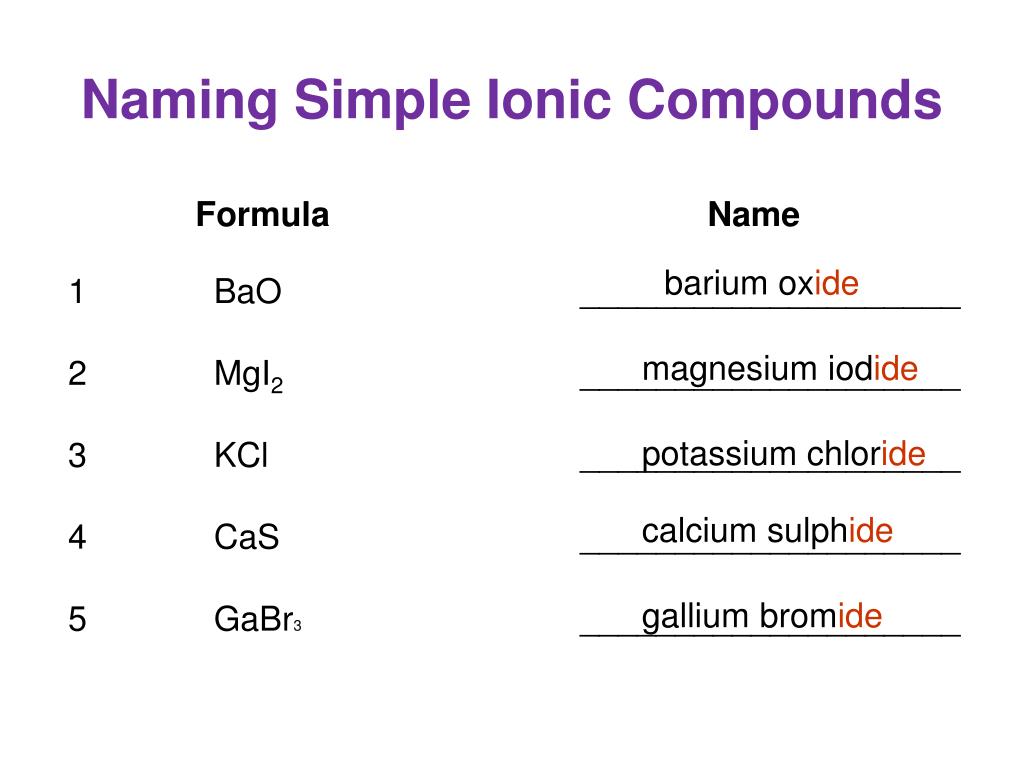
Magnesium Oxide Ionic Compound Formula . Why is this the case? magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. We will start with binary ionic,. The magnesium ion is an alkaline. Magnesia brick is made from periclase, the. — the ionic formula for magnesium oxide is mgo. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. This is two positive charges and two negative charges. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
from www.slideserve.com
The magnesium ion is an alkaline. — the ionic formula for magnesium oxide is mgo. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. Why is this the case? Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. We will start with binary ionic,. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. This is two positive charges and two negative charges.
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496
Magnesium Oxide Ionic Compound Formula Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. The magnesium ion is an alkaline. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. Why is this the case? We will start with binary ionic,. Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. This is two positive charges and two negative charges. magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. — the ionic formula for magnesium oxide is mgo. Magnesia brick is made from periclase, the. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities.
From guidelibperplexing.z13.web.core.windows.net
Diagram Of The Formation Of An Ionic Compound Magnesium Oxide Ionic Compound Formula — the ionic formula for magnesium oxide is mgo. Why is this the case? Magnesia brick is made from periclase, the. Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. The magnesium ion is an alkaline. We will start with binary ionic,. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth. Magnesium Oxide Ionic Compound Formula.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download Magnesium Oxide Ionic Compound Formula magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. The magnesium ion is an alkaline. Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. We will start. Magnesium Oxide Ionic Compound Formula.
From studylib.net
Ionic Compounds Naming Magnesium Oxide Ionic Compound Formula We will start with binary ionic,. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. Why is this the case? you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. The magnesium ion is an alkaline. — the. Magnesium Oxide Ionic Compound Formula.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Magnesium Oxide Ionic Compound Formula Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. The magnesium ion is an alkaline. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula.. Magnesium Oxide Ionic Compound Formula.
From www.slideshare.net
Chem matters ch6_ionic_bond Magnesium Oxide Ionic Compound Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Why is this the case? Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. The magnesium ion is an alkaline. — the ionic formula for magnesium oxide is mgo. We will. Magnesium Oxide Ionic Compound Formula.
From studylibraryopinion.z14.web.core.windows.net
How To Write Formula For Ionic Compounds Magnesium Oxide Ionic Compound Formula We will start with binary ionic,. — the ionic formula for magnesium oxide is mgo. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.. Magnesium Oxide Ionic Compound Formula.
From studylib.net
Determining the Empirical Formula of Magnesium Oxide Magnesium Oxide Ionic Compound Formula This is two positive charges and two negative charges. magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. when an ionic compound is formed from magnesium and oxygen, the. Magnesium Oxide Ionic Compound Formula.
From www.slideserve.com
PPT Ch. 7 (Con’t.) Formula Writing & Naming of Compounds PowerPoint Magnesium Oxide Ionic Compound Formula This is two positive charges and two negative charges. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Why is this the case? magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. Magnesia brick. Magnesium Oxide Ionic Compound Formula.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot Magnesium Oxide Ionic Compound Formula We will start with binary ionic,. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. — the ionic formula for magnesium oxide is mgo. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities.. Magnesium Oxide Ionic Compound Formula.
From wiredatapoliciaj0.z22.web.core.windows.net
Mg Dot Diagram Magnesium Oxide Ionic Compound Formula you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the. Magnesium Oxide Ionic Compound Formula.
From www.youtube.com
Formation of Magnesium Oxide (MgO) Chemical Bonding Atomic Magnesium Oxide Ionic Compound Formula We will start with binary ionic,. Why is this the case? The magnesium ion is an alkaline. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. Magnesia brick is made from periclase, the. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular. Magnesium Oxide Ionic Compound Formula.
From www.toppr.com
Magnesium Oxide Formula Definition, Concepts and Examples Magnesium Oxide Ionic Compound Formula magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. when an ionic compound is. Magnesium Oxide Ionic Compound Formula.
From webapi.bu.edu
😝 Write the formula for the compound magnesium oxide. What is the Magnesium Oxide Ionic Compound Formula Magnesia brick is made from periclase, the. magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. when an ionic compound is formed from magnesium and oxygen, the magnesium. Magnesium Oxide Ionic Compound Formula.
From www.showme.com
Formula for Magnesium oxide Science, Chemistry ShowMe Magnesium Oxide Ionic Compound Formula Why is this the case? We will start with binary ionic,. — the ionic formula for magnesium oxide is mgo. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. The magnesium ion is an alkaline. Basic refractories include magnesia, dolomite, chrome, and combinations of these materials.. Magnesium Oxide Ionic Compound Formula.
From www.flinnsci.com
Ion Names, Formulas, and Charges Charts for Chemistry Magnesium Oxide Ionic Compound Formula magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. The magnesium ion is an alkaline. — the ionic formula for magnesium oxide is mgo. We will start with binary ionic,. magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium. Magnesium Oxide Ionic Compound Formula.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Magnesium Oxide Ionic Compound Formula Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. Why is this the case? when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom. Magnesium Oxide Ionic Compound Formula.
From testbook.com
Magnesium Oxide Formula Check Structure, Preparation & Uses Magnesium Oxide Ionic Compound Formula magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. We will start with binary ionic,. Why is this the case? magnesium oxide is the oxide salt of magnesium with antacid, laxative and vascular smooth muscle relaxant activities. Magnesia brick is made from periclase, the. when an. Magnesium Oxide Ionic Compound Formula.
From www.numerade.com
SOLVED 'Which diagram shows the correct way to represent an ionic Magnesium Oxide Ionic Compound Formula Magnesia brick is made from periclase, the. magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. This is two positive charges and two negative charges. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. Magnesium Oxide Ionic Compound Formula.